In the
end of the semester, through Information and Communication Technology (SSI 3013)
lessons, I have learn many new things. My first lesson is about Paint. Actually
I am not familiar with Paint. But after I heard about Mr. Azmi explanation, I am
able to do my work with Paint now. Then, blog. Actually I’ve been to a blog
workshop before. However, there’s not many that I learned from there. But,
through En. Azmi assignment, make a blog to publish his assignments and others,
I manage to make a blog successfully and know many new things about blog. The assignments
about Smart School really gives me many useful information related to education
and Smart School development. This course
not only teach me about ICT but about learning and teaching process and also about
education progress in Malaysia. Through this
course too, I manage to learn about many interesting thing related to education
such as teaching tools and others. This course also teach me about cooperation
within group, division of time to accomplish many works and many more. For me, I
think the class session is okay however I think the environment is quite lame
and not happening. However, this might be due to the class session which is at
the evening thus the student is tired hence make the environment become lame.
Eportfolio
Monday, 3 December 2012
Sunday, 2 December 2012
Essay Data Logging
Title
Data Logging On Determination
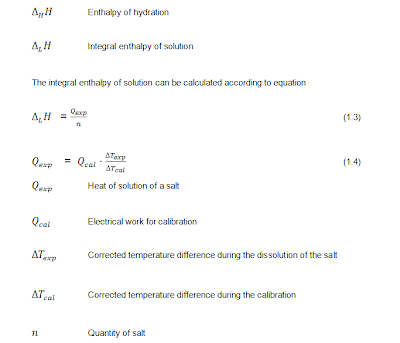
The Hydration Enthalpy Of An Electrolyte.
Introduction
What
is Data Logging?
Data logging is
the process of using computer to collect data through sensors, analyze the data
and save the output the results of the collection and analysis. Data logging is
also implies the control of how the computer collects and analyzes the data. Data
logging is commonly used in scientific experiments and monitoring systems where
there is the need to collect information faster than a human can possibly
collect the information and in this case the accuracy is essential. Examples of
the types of information and a data logging system can collect include
temperatures, sound frequencies, vibrations, times, light intensities,
electrical currents, pressures and changes in states of matter. Computer data
logging has been used in teaching science in number of countries since the
1980s. As summary, a collection of results is known as data while the process of handling data by using modern computer
technology referred to as data logging.
Elements
of Data Logging
Data logging
system consists of sensor, interface box and computer with appropriate
software. A sensor is a device that
responds to some physical property of the environment like temperature,
pressure, light intensity, voltage, current and many more. Then, the variation
of physical properties is converted into signals that is recognized by a device
called interface box. The interface box
is used to convert the signal of the sensor to a digital signal which is can be
read by the computer. The interface will connected to the computer via a serial
port of computer. Then, computer is
used to display the data and read the information from sensor. Specialised
computer software is required so that the computer can interpret and process
the signals from the interface box. Examples of data logging software such as
Data Studio, HOBOware and many more. The sensors, interface box, computer and
appropriate computer software called as the data logging system.
Advantages
the Use of Data Logging in Science Teaching
From research
that have been carried out, data logging can improves graphing skills of students and help them to form links
between what they have learnt in class with the process of investigating
scientific relationships. The interpretations of graphs is significantly
improved as they have applied the skills of real-time reporting where the graph
is drawn at the same time as the experiment is performed will encourage
reflection and interpretation among students. Apart from that, computer data
logging practical work also will give more benefits compared to the
conventional approach in the presentation of data. By using data logging, the quality of written homework was greatly
improved as the data can be easily manipulated and presented in the form of
clearly drawn graphs. Real-time data logging presents the graph on the screen
“as it happens” and this is especially beneficial to the less able student.
Data logging also can save times in pattern of student’s activity in preparing apparatus
and materials, measuring and reporting data towards spent more on observation,
manipulation of data and discussion among students of the results obtained when
using this data logging system. The automatic logging of experimental data and
graphical representations allowed for more focused approach to changes in
experimental variables and discussion of results. It was clear that they have
better insight into this experimental work being performed. Besides, students need to take less prolonged
readings and through the software they can spend more time in analysing
information. The immediate visual feedback via the computer enables ‘on the
fly’ adjustment to experiments. In addition, without the aid of computer data
logging spent more considerably more time in data collection.
In general, we can see that students find information
technology to be a good stimulus for learning. The software tools for
calculation and analysis will reduce tasks considered to be tedious and
repetitive into creative opportunities for carrying out investigation in
laboratory. This will increased level of
interest among students in bringing science teaching and learning process
into twenty-first century. Data logger also allows students to collect data from whole range of sources at
one particular time. For example, in evaporation experiment they are not
just only measuring the temperature today, but three or fours variables that
might be affecting the outcome of evaporation. Students are able to collect
information anywhere and anytime, which means a whole community potentially
becomes part of the learning environment.
Disadvantages
the Use of Data Logging in Science Teaching
One of the disadvantages of using data logging is the
special features of data logging graphing software sometimes gives the
variaties of difficulties associated in handling the data logging software,
They need to setup the software more wisely and carefully so that it does not
gives not accurate and not precise result when it was displayed on the
computer. If it was happened, they need to modifiy any devices related so that
it can be function well.
Besides, by using data logging, the students will face
difficulties if the devices or equipments are broken or cannot be function
well. So, they need times in repairing the devices so that they can use to run
their experiment. The data logging and computerized devices really need
meticulous care as there are really sensitive tools.
Experiment
: Determination Of The Hydration Enthalpy Of An Electrolyte
Theory
The dissolution of a solid
electrolyte in water is primary determined by two simultaneously occur in
processes : the destruction of the crystal lattice and the hydration of the
ions. The degradation of the crystal lattice is an endothermic process because
energy is required to breakdown the chemical bonds, whereas the hydration of
the ions is exothermic. Depending on the type of lattice, and both the radius
and the charge of the ions (charge density), the resulting enthalpy of the
solution can be either or exothermic. When a salt exists in both hydrated and
dehydrated forms, and one assumes that during the dissolution of the hydrated
salt only the degradation of the crystal lattice occurs, the enthalpy of
hydration can be calculated with Hess’s theorem (Figure 1).
|
|
I.
Engage
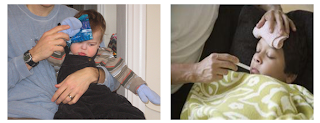
Picture (a) is
about the process melting of an ice while picture (b) is about the process of
freezing of an ice. In processes, heat or energy is involved. But, there are
some different either heat absorbed or heat released. Reaction that release
heat termed exothermic while reaction that absorbed heat termed endothermic.
Which one of this picture is exothermic reaction? Which one of this picture
endothermic reaction? How the temperature or heat of the surrounding changes?
In this
experiment, anhydrous copper (II) sulphate and copper (II) sulphate were used.
Both compounds are made up of copper and sulphate but one of them contain water
molecule while the other one do not contain water molecule. Most of the people
always have misconception about the molecular formula of copper (II) sulphate
and anhydrous copper (II) sulphate. Most of them think that the molecular
formula of anhydrous copper (II) sulphate is CuSO4.5H2O
while the molecular formula of copper (II) sulphate is CuSO4.
Actually,
anhydrous copper (II) sulphate is molecule without molecule of water but copper
(II) sulphate is molecule with water. So, the actual molecular formulae of
anhydrous copper (II) sulphate is CuSO4 while the molecular formula
of copper (II) sulphate is CuSO4.5H2O But, is both of
them have same type of enthalpy? If both of them have same type of enthalpy,
which one of them has highest enthalpy value? Which one undergoes exothermic
process and which one undergo endothermic process?
Engage is the
process to generate idea or to induce any idea about the topic. The teacher can
use the phenomenon happen in our life, video simulation or figures and pictures
to make the students able to think why it happened. In this experiment, we have
used the formation of ice and the melting of ice to relate both of these
situations with endothermic and exothermic reaction so that the students can
start to think. In engage stage, the students will start to explore about the
topic, start to think abstractly and start to formulate and develop the concepts.
II.
Empower
Method
1)
First, the experiment set-up is
performed.
2)
Then, 24.97g (0.1 mol) of copper (II)
sulphate and 15.96g (0.1 mol) of anhydrous copper (II) sulphate, which has been
finely pulverized in a mortar are weighed.
3)
The calorimeter is filled with 900mL
of distilled water. The magnetic stirring bar is put into the calorimeter and
latter is placed onto heating stirrer.
4)
After attaching the heating coil and
the temperature probe, the magnetic stirrer is switched on. Please be aware, do
not mistakenly switch on the heating unit.
5)
When the equilibrium temperature has
been reached in the calorimeter approximately 5 minutes, the first copper salt
is added to the water by pouring it through powder funnel which has been inserted
in the opening in the lid. While doing so, make sure the entire quantity of
salt is added to the water without any loss.
6)
The registration of the
temperature-time curve is begun first.
7)
Ten minutes after the salt has completely dissolved,
the electrical calibration* is conducted in order to determine the total heat
capacity of the calorimeter.
*10AV is supplied to the
work and power meter for the electrical heating. Performed a reset and then the
free ends of the heating coil’s connection cables are plugged into the output
jacks of the work and power meter. The system is heated continuously, and the
supplied quantity of energy is measured. After electrical energy amounting to
approximately 4000Ws has been supplied, the heating is switched off and the
exact quantity of electrical energy is read from the meter.
8)
10 minutes later the temperature recording is
also terminated.
9)
The corrected temperature differences,
∆T for the calibration and solution experiment are determined are determined as
shown in Figure 2.
10)
This correction is necessary because
of the heat exchange with the surroundings. The vertical straight line
which intersects the lines
and
are drawn in such a manner that the shaded
areas are equal size.
11)
For the calibration,
is determined analogously from the
intersection points of
.
12)
The same experiment is repeated to
determine the enthalpy of solution for both Copper (II) sulphates. At least two
measurements should be performed for each salt to avoid errors and to be able
to calculate the mean value.
Figure 2 : Graphical
determination of the correct
T
values from the temperature time curve.
Figure 1 : Apparatus
set up
Figure
2 :Picture of sensor
Discussion.
·
Hydrates are compounds
that incorporate water molecules into their fundamental solid structure.
·
All hydrating water is removed, the material is said to be
anhydrous
·
Enthalpy of solution of copper (II)
sulphate is exothermic reaction
·
Enthalpy of anhydrous copper (II)
sulphate is endothermic reaction
· The 5H2O
in the formula of anhydrous copper(II) sulphate is called the water of crystallisation
and forms part of the crystal structure when copper(II) sulphate solution is
evaporated and crystals form. This
crystal structure is broken down on heating and the water is given off. So, the
thermal decomposition is endothermic as heat is absorbed to drive off the
water. Meanwhile, the reverse reaction is called as and exothermic
reaction. This is because it needs adding water to white anhydrous copper(II)
sulphate and the mixture heats up as the blue crystals reform. The reverse reaction is used as a
simple chemical test for water where white anhydrous copper(II) sulphate turns
blue.
·
The enthalpy change is the
‘enthalpy change of hydration’.
·
Enthalpy change of reaction is
endothermic reaction.
·
The value of enthalpy change of
reaction is 152.04kJmol-1.
·
CuSO4
(s) + 5 H2O (l) →
CuSO4 • 5H2O (s)
·
CuSO4 (s) + H 2O(l) →
CuSO4 (aq)
(ashy white) (deep blue)
Empower is something that gaining a power in particular activity by
individuals or groups. It is also the process of giving power to the students
or process that foster and facilitate their taking of power. Besides, empower
also a process to achieve goals or some effort to understand some critical
understanding. So, in data logging learning, the teacher can empower their
student by performing an experiment so that they can have more understanding
about what they have learnt theoretically in class. From data logging process,
the method used is by using a sensor and computer rather than using traditional
method. So, the students can see clearly the result. Hence they can make
comparison directly between their results with the theory. If there’s any
differences happen, they can make discussion regarding the result they got. The
students will construct their concept of learning by understanding the process
involve in the experiment. In this topic, students may be
surprised that energy can either be evolved or absorbed in reactions. To make a
chemical bond, another bond must first be broken. It is the sum of the energy
changes in making and breaking bonds that results in the overall energy change. If temperature sensors and data logging equipment are available,
they may be appropriate in this context.
This is because a temperature sensor attached to
a computer can be used in place of a thermometer. It can plot the temperature change on a graph and make a
helpful demonstration to the students of what happens when chemicals react. This data logging set up might be the
basis for a project where students have
to find the mix of chemicals that yield the optimal heat loss or
gain.
gain.
III.
Enhance
Cold packs and putting ice in
towel causes a cooling effect on their person’s head and temporarily relieve
the pain and fever. Explain.
There are two
types of cold packs which are small inner bag and an outer bag. The small inner
bag can be just water and the outer bag can be ionic salts such as Ammonium
Chloride or Potassium Nitrate. When the pack is squeezed, the small inner bag
breaks the ionic salt dissolves in water. When the cold pack is used, the
chemicals inside the pack are made to react with each other and this reaction
is highly endothermic in nature. Endothermic
reactions involve the absorption of heat. The ammonium nitrate mixing with the
water creates cold. The temperature of cold packs can reach back to normal
temperature. The heat energy is taken into the system from the
surrounding. The surrounding in this case is the person’s head.
Another
alternative way in reducing headache or fever is by wrapping some ice inside a
small towel and hold it against forehead. This is a traditional way practiced
by our parent to cool down the temperature and pain. The concept is the same as
the cold pack. As the ice melts to become liquid water, it would take in energy
from the surroundings and melting is considered an endothermic process. The
solid ice will become the system and our forehead becomes the surrounding. The
system takes in heat energy from the surrounding and thus directly remove heat
from our forehead and can reduce pain.
Enhance is
something to make it better, to add or contribute to. In data logging learning,
enhance is the third phase after empower stage which is to increase student’s
understanding on a given problem or topic by relating it with the example of
application. For example, if we give some situation or application in our
environment or daily life application, the students will be able to relate the
reasons of the situation given on what they have learnt. So that, they can
apply their learning concept in daily life application in order to enable them
to understand and remember. So, in this experiment, we should provide an
example related to endothermic or exothermic reaction. The students may have
developed their understanding about these reactions at empower stage but then
when at the enhance process, they just need to add any additional knowledge by
relate in with daily life applications.
Extension
N2 (g) + 3H2
(g) ↔ 2NH3 (g)
What will happen to the
production of ammonia gas reaction if we increase the concentration the
temperature of the reactant mixture?
If the temperature of a
reaction mixture is increase, the equilibrium will shift to decrease the
temperature. Based on Le Chatelier’s Principle which stated that if a chemical
system at equilibrium experiences a change in concentration, temperature or
total pressure, the equilibrium will shift in order to minimize that changes a
new equilibrium is established. So, if we increase the temperature, the
equilibrium will shift to the reactant part which is left. So, the reaction
will undergo endothermic reaction as it use up heat energy. Ammonia will broken down into hydrogen and
nitrogen gas. An increase in temperature will decrease the yield of ammonia ,
NH3.
Conclusion
As a teacher,
we should apply the use of data logger in teaching and learning process in line
with the developments of technology. The use of this type of teaching process
can enhance the learning style and gives the positive impact on teaching
Science process especially. Teacher will use the three steps in data logging
process like engage, empower and enhance to make the students get highly
understanding about what they have learnt. Usually during science theory
classes, students do not have the opportunity to
verify the appropriateness of the information that
the teacher is putting forward. So, the use of technology which incorporates
data logging will significantly ease the situation and able to develop logical
understanding of the abstract concept. At the same time, they can obtain the
information on the truthfulness of the underlying processes. The use of data
logging will bring cognitive acceleration to learning, as the teachers can
support their teaching with undeniable facts, thus students can be able to
revisit any misconception they hold on the spot.
References
A.Gras-Velazquez,
A.Joyce and M. Le Boniec. Impact of Data Loggers on Science Teaching and
Learning. Retrieved on 27 Dec. 2012 from http://files.eun.org/netbooks/ACER_Fourier_EUN_Science_pilot_report_2012.pdf
Data Logger. Retrieved on 27 Dec. 2012 from en.wikipedia.org/wiki/Data_logger
Declan Kennedy (2000). The Use of Data Laogging in
Teaching Physics and Chemistry in Second-Level Schools in Ireland. Retrieved on
27 Dec.2012 from http://www.outlab.ie/forums/documents/the_use_of_datalogging_in_teaching_physics_and_chem_in_second_level_schools_report_ie_111.pdf
Engaged Learning. Retrieved on 29 Dec. 2012 from
Lorraine
Stefani (2008). Engaging our Students in the Learning Process : Points for
Consideration. Journal of International Journal for the Scholarship of Teaching
and Learning. Vol. 2, No. 1. Retrieved on 28 Dec. 2012 from http://academics.georgiasouthern.edu/ijsotl/v2n1/invited_essays/Stefani/Invited_Essay_Stefani.pdf
Using ICT and Data Logging in Teaching and Learning of
Science. Retrieved on 27 Dec.
Technology-integrated Science Teaching. Retrieved on 28
Dec. 2012 from
Subscribe to:
Comments (Atom)